A local partner that is responsive, accountable, experienced
0
Thermo Fisher facilities in PA
0
PA biotech studies since 2019
0
PA biotech clients since 2019
0
Number of employees in PA
In biotech, it’s vital to anticipate every milestone and change in direction.
We share your passion for bringing novel therapies to market — and your sense of urgency. Every milestone. Every shift in direction. Every decision is critically important to successfully achieving your goals.
PPD™ Biotech solutions offer local, experienced teams and scalability of a global CRO, combined with a hands-on approach that provides clinical expertise and strong operational delivery every step of the way.
We have boots on the ground in Pennsylvania and this local presence ensures that our resources are easily accessible to you.
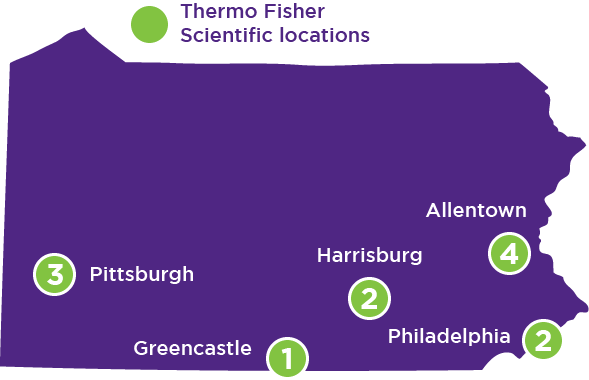
Our presence in Pennsylvania
From the City of Bridges to the City of Brotherly Love, our offices span across Pennsylvania to provide you local experience and accessibility.
Our Thermo Fisher Scientific locations include the Pittsburgh, Greencastle, Harrisburg, Allentown, and Philadelphia metro areas.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
STEM event where Thermo Fisher Scientific colleagues showed strawberry DNA to 3rd and 4th graders at a school in PA.

Community Engagement
Public STEM fair in PA, during which Thermo Fisher Scientific colleagues led lava lamp and dry ice demonstrations.

Community Engagement
Public STEM fair in PA, during which Thermo Fisher Scientific colleagues led lava lamp and dry ice demonstrations.
PPD Biotech model
With PPD Biotech solutions, we provide end-to-end capabilities, economies of scale, and greater clinical trial reliability and quality, while simplifying your drug development journey.
We intentionally built a model to better serve small- to mid-sized biotech and pharma companies by making the line between internal and outsourced teams indistinguishable.

Collaborating with a provider of CRO solutions
As a trusted extension of a drug developer’s team, contract research organizations (CROs) have the capacity to complete a range of day-to-day research activities that are either not possible or too expensive to achieve in-house.

Ensuring patient diversity
Diverse patient populations in clinical trials ensure that safe and effective therapies are brought to market. To meet your patient diversity goals and regulatory requirements, we recommend partnering with a CRO that has a demonstrated history of implementing comprehensive diversity plans.

Therapeutic area spotlight
Thermo Fisher Scientific is a CPRIT-approved partner. With experience in more than 700 hematology and oncology clinical trials, the PPD clinical research business of Thermo Fisher Scientific has expertise across the full range of cancer therapies — from immuno-oncology and targeted therapies to novel and emerging therapies, including cell and gene therapies.
In the past five years alone, we have supported 34 oncology drug approvals in the U.S. — including three first-in-class products.
Videos
Your commitment to developing meaningful solutions to neuroscientific health challenges is shared by our experts.
In the past five years, we have supported more than 450 clinical studies in neuroscience and over 50 rare neurological disorder studies, including over 60,000 patients and 9,000 sites around the globe.
- Relieve caregiver burden in neurodegenerative trials
- Case Study: How EmVenio and PPD delivered home visit services for Alzheimer's patient
- Article: Preparing for growth in neurodegenerative treatment opportunities
- Brochure: Accelerate your gene therapy trial
Visit us in October at American Academy of Ophthalmology (AAO) 2024
Our therapeutic area expertise spans several large drug disease markets along with growing areas of R&D innovation.
In the past five years, we have supported 9 of the 10 top-selling drugs.
Functional service partnerships (FSP)
Our FSP models increase flexibility and provide access to the exact expertise your project needs, when you need it, in just the right amount.
Let us help you advance your next drug development project — whether you need to fill small gaps in services or support large-scale programs with dedicated capacity management across functions.

Connect with us

Danko Dominis, M.D., Ph.D.
Regional Medical Officer
Learn more about Danko >
- 20+ years of experience in the clinical & pharmaceutical industries
- CRO management roles in NA, EMEA, and APAC with medical and commercial responsibilities
- Experience in Rare Diseases, Oncology, Respiratory, Women’s Health, and Endocrinology
- Medical training in Obstetrics & Gynecology, Ph.D. thesis in Endocrinology

Paul Roberts, Ph.D., Senior Director
Biotech Portfolio Lead
Learn more about Paul >
- 18+ years of project and clinical management experience in clinical research; joined PPD in 2012
- Global experience across all trial phases
- Therapeutic experience in Oncology, Hematology, Infectious Disease, Musculoskeletal, Neurology, Respiratory, NHV, Genitourinary, and Metabolic trials
- SME in Early Phase Oncology patient trials and in Clinical Imaging
- Ph.D. in Cell and Molecular Biology

JP Lewis
Account Development Lead
Learn more about JP >
- 10+ years of business and customer service-oriented experience
- Priority on cultivating and maintaining relationships based on mutual trust and understanding
- Focus on staying informed on industry trends and developments to best align PPD’s capability with client needs

Michael Eveland
Executive Director, Biotech
Business Development
Learn more about Michael >
- 11+ years in the CRO industry, 2+ years at the PPD clinical research business of Thermo Fisher Scientific
- Responsible for global clinical development services for Phase I-IV clinical trials
- All major therapeutic areas including Oncology, Neuroscience and pain, Immunology, Infectious Disease, Respiratory, Rare Disease, Hepatology and Gastrointestinal